Tuberculosis Blood Test
By Hopkins Medtech
About Tuberculosis in the US
13-15 million people in the United States live with Latent Tuberculosis Infection (LTBI). Almost half of the US population (42%) with LTBI are untreated and can infect 10 to 15 additional people per year.
The CDC launched the ‘Think. Test. Treat’ TB campaign to screen high risk individuals for LTBI. An aggressive US strategy to develop new tools for TB diagnosis, treatment, and prevention is needed.
How does TB-IGRA fight Tuberculosis?
Interferon Gamma Release Assay (IGRA) is a whole blood test that can aid in diagnosing Mycobacterium tuberculosis infection. The test measures a person’s immune reactivity to M. tuberculosis. T lymphocytes from most persons that have been infected with M. tuberculosis will release interferon gamma (IFN-γ) when mixed with antigens (substances that can produce an immune response) derived from M. tuberculosis.
With high sensitivity and specificity, TB-IGRA can enable early treatment and prevention strategies that are crucial in reducing the global burden of tuberculosis.
Understanding Wantai TB-IGRA (RUO)
Wantai TB-IGRA (RUO) is the Only Other IGRA Endorsed by the WHO. With easy steps and high performance, we provide the WHO recommended Wantai TB-IGRA RUO test that aids in diagnosis of TB infection.
With easy steps and high performance, we provide the WHO recommended Wantai TB-IGRA RUO test that aids in diagnosis of TB infection.

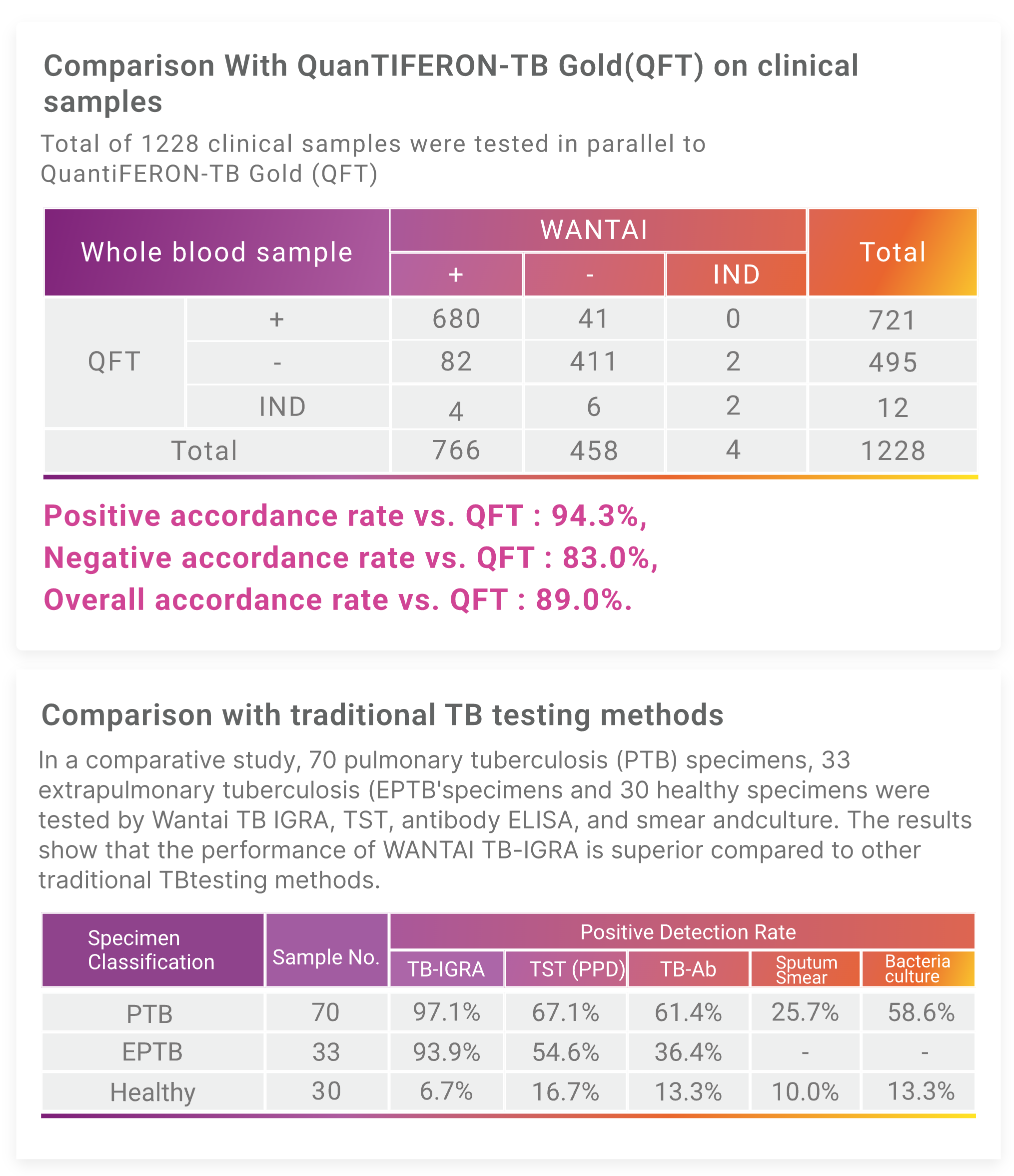
Wantai TB-IGRA Performance
High accordance with QuantiFERON-TB Gold (QFT-G) on clinical samples.
Specificity of 94.8% and sensitivity of 81.0%.
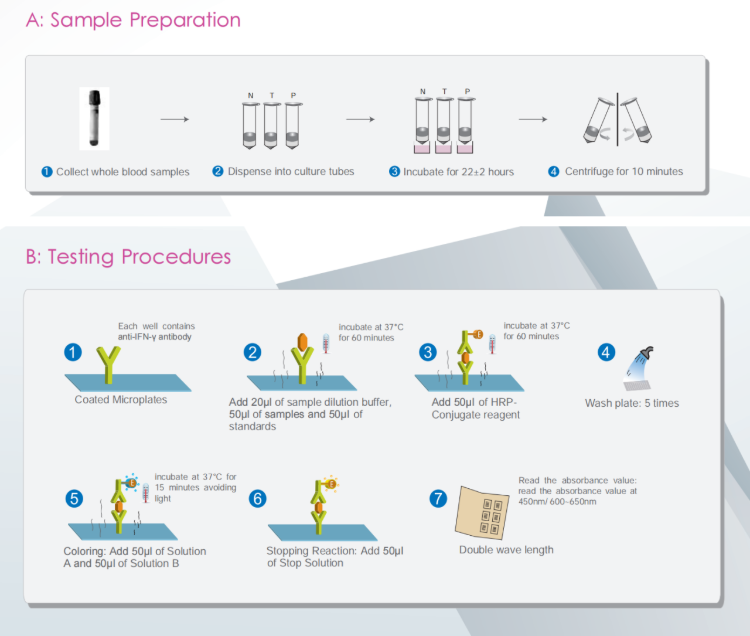
How to use Wantai TB-IGRA (RUO)?