FDA issuing Final Rule on regulating LDT
FDA has issued its Final Rule on regulating Laboratory Developed Tests (LDTs), fulfilling its promise earlier this year despite facing considerable opposition. The Final Rule, announced on April 29, has stirred controversy across various sectors.
Previously, the FDA faced opposition from various quarters when it announced plans to regulate LDTs. The reasons for opposition included concerns that strict regulation would stifle innovation in the testing industry, doubts about the FDA’s capacity to regulate the millions of tests conducted across the United States, and concerns from test manufacturers about increased certification costs and difficulties.
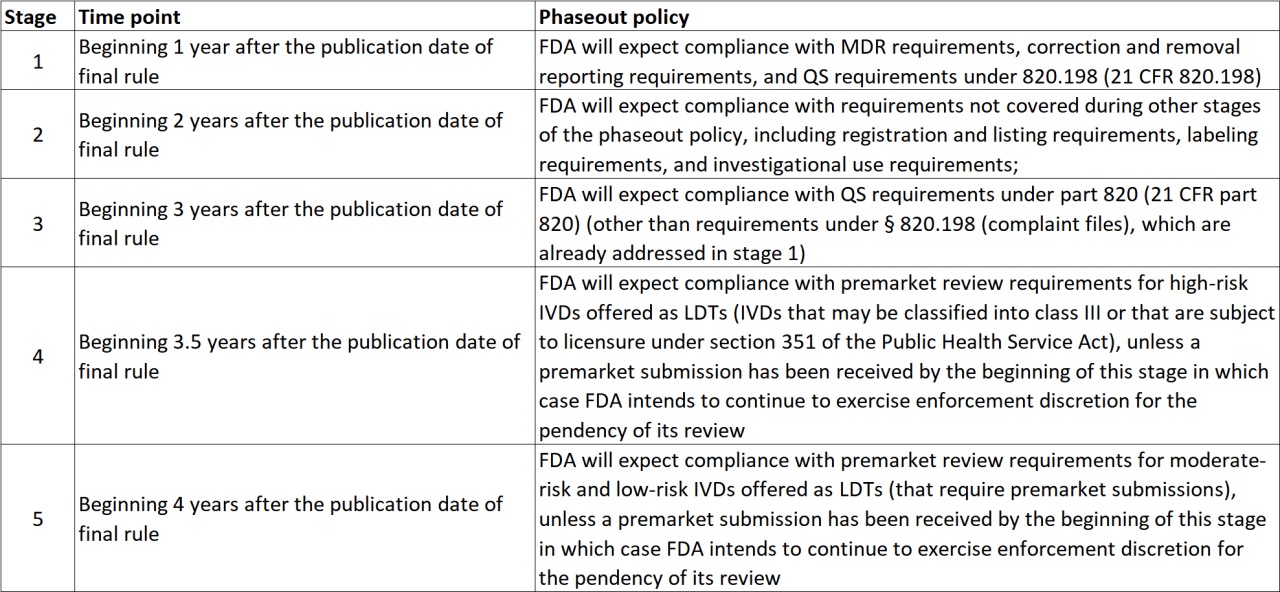
In the newly issued Final Rule, the FDA emphasized that it had considered numerous opinions from stakeholders but remained committed to reforming LDT regulation. The FDA argued that the current lack of regulation in testing has led to industry confusion. To help the testing industry adapt, the FDA proposed a four-year transition period.
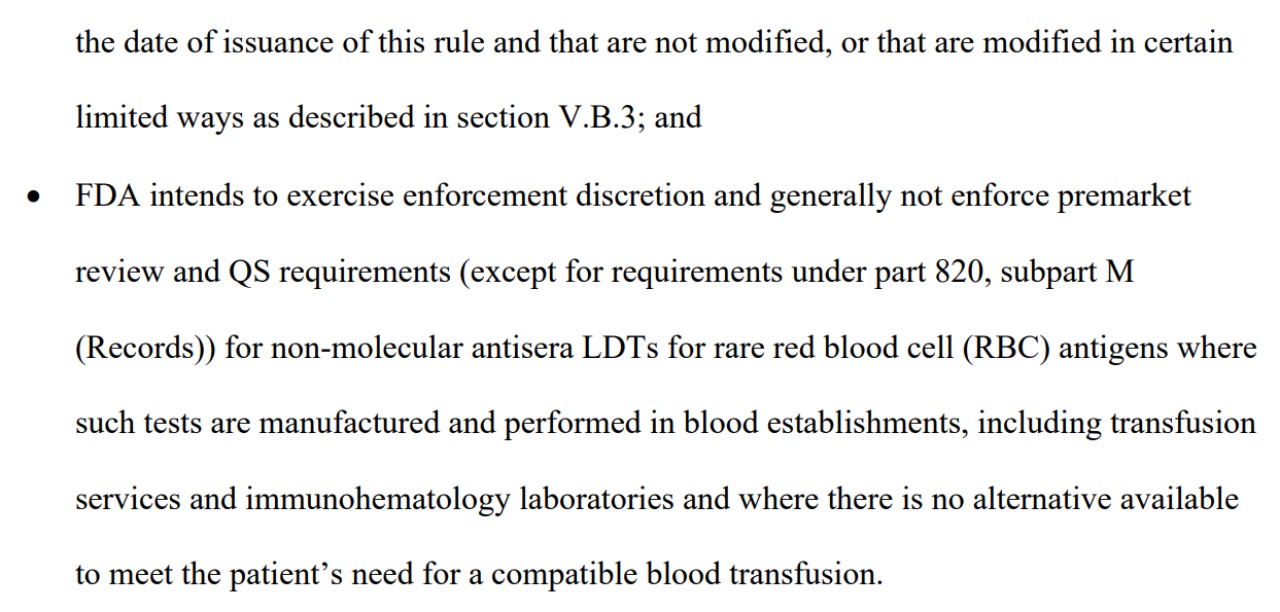
Additionally, the Final Rule highlighted several crucial points. The FDA listed new areas where it would continue to exercise Enforcement Discretion, meaning it would not enforce certain regulatory requirements for LDT laboratories. These areas include tests conducted by the Veterans Health Administration or the Department of Defense, LDTs approved by the New York State Clinical Laboratory Evaluation Program (CLEP), and labs integrated with healthcare systems to address unmet testing needs.

Another key point is the FDA’s plan to downgrade some Class III tests to Class II, which would reduce certification costs and time for IVD manufacturers. This downgrade is expected to occur in Phase 4, four years after the Final Rule’s implementation.
Following the release of the Final Rule, the Association for Diagnostics & Laboratory Medicine (ADLM) and the Association for Molecular Pathology (AMP) expressed dissatisfaction and called for Congress to pass the VALID Act. AMP even proposed regulatory initiatives regarding LDTs.
Although some compromises are evident in the FDA’s Final Rule, its implementation could significantly impact laboratories in the United States. Large laboratories with tests approved by CLEP stand to benefit, as they will incur fewer ongoing approval costs. On the other hand, smaller laboratories or those without CLEP approval or integration with healthcare systems may be more affected.
The Final Rule will be further explained in a webinar scheduled for May 14.
Despite the FDA’s new Final Rule, its ultimate passage still depends on the United States Congress. Public hearings, such as the one organized by the House Energy and Commerce Committee, have been held to assess the impact of FDA proposed rules. Additionally, Republican Representative Cathy McMorris Rodgers has called on the FDA to abandon the Final Rule, citing concerns about its impact on patient care.
Therefore, whether the FDA’s Final Rule will ultimately pass remains to be seen.