Roche receives FDA Breakthrough Device Designation. Why is pTau-217 is becoming a hot biomarker for Alzheimer’s Disease?
We previously discussed that Leqembi, an Alzheimer’s disease treatment drug received FDA approval in January last year, through an accelerated pathway. This gave hope as offering a treatment option for Alzheimer’s disease. This marks a beginning of 2023 to become a year with explosive growth in Alzheimer’s blood tests.
This year, Alzheimer’s blood testing continues to intensify. On Thursday, Roche announced that its Elecsys pTau-217 plasma biomarker test has received FDA Breakthrough Device Designation. This test marks Roche’s first FDA breakthrough device designation since its collaboration with Eli Lilly in March 2023. Roche’s previous FDA breakthrough device designation in 2022 was for Elecsys pTau-181 and ApoE4 immunoblood tests. Roche currently has two other tests FDA certified: Elecsys beta-Amyloid (1-42) CSF II (Abeta42) and Elecsys Total-Tau CSF (tTau) assays, both of which test cerebrospinal fluid, a more complex and painful sampling method for patients.
pTau-217 has recently become a highly sought-after biomarker for Alzheimer’s disease. Just the day before yesterday, Quanterix CEO Masoud Toloue stated that the growth momentum of pTau-217 as an Alzheimer’s disease marker prompted Quanterix to protect its intellectual property rights (patent number: 11275092). Quanterix’s research on pTau-217 is also conducted in collaboration with Eli Lilly.

However, pTau-217 has already been commercialized by multiple testing companies. Apart from Roche, companies like LabCorp, Neurocode, and C2N Diagnostics have launched their blood pTau-217 tests, while Quest also plans to launch a pTau-217 plasma test in the second half of this year.
Why has pTau-217 become such a hot biomarker for Alzheimer’s disease?
Currently, the mainstream biomarkers for Alzheimer’s blood tests include Aβ42/40, pTau-181, pTau-217, and pTau-231.
Aβ42/40: Although Roche has commercialized cerebrospinal fluid Aβ42/40 testing, detecting Aβ (β-amyloid) in blood is challenging. While Aβ is also produced peripherally, its concentration does not accurately reflect central nervous system amyloid accumulation. Despite Quest’s launch of Quest AD-Detect using blood Aβ42/40 last July, the lack of peer-reviewed literature on this method led to its removal from the market last month.
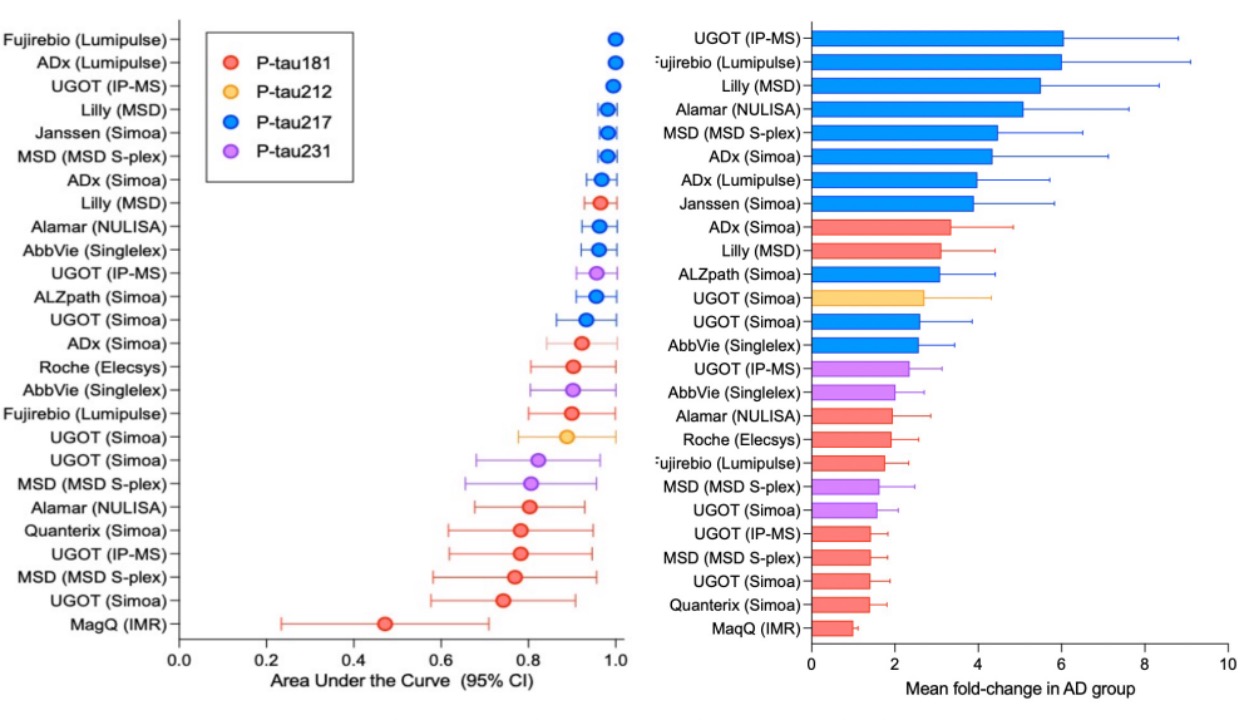
pTau-181, pTau-217, and pTau-231: Currently, over 80 pTau markers are under study, with pTau-217 and pTau-181 considered the most promising biomarkers, while pTau-231 may serve as a state marker, but this requires further validation. In a prospective study cohort, pTau-217 showed stronger correlation with Aβ positivity compared to plasma pTau-181, pTau-231, N-terminal tau, GFAP, or NfL. Furthermore, preliminary evidence suggests that pTau-217 levels increase in the preclinical stages of Alzheimer’s disease and are associated with brain Aβ burden. When distinguishing between different types of neurodegenerative disease pathology, both pTau-181 and pTau-217 perform well, but pTau-181 seems less accurate than pTau-217 in differentiating Alzheimer’s disease from other forms of neurodegenerative diseases.
Interestingly, a study initiated by the Global Biomarker Standardization Consortium compared multiple pTau-217 assays from 11 different manufacturers. Although the sample size was small, pTau-217 showed a higher AUC than pTau-181 and pTau-231. This finding is reassuring to industry experts, as it suggests several reliable pTau-217 plasma tests are available.
Returning to Quanterix’s decision to patent pTau-217, Duke University law professor/intellectual property expert Arti Rai stated that establishing patent infringement claims for tau protein quantification levels would be challenging because it resembles a natural law, which cannot be patented, but laboratory testing methods can be patented.
This move may affect Roche, LabCorp, and Quest, but not C2N, as they use mass spectrometry for detection. If Quanterix indeed wields the patent weapon and challenges these major players’ shares of this huge market of Alzheimer’s Disease blood testing, the road to patent protection may not be so easy. After all, Alzheimer’s testing is currently a fiercely contested battleground.